COVID-19
Vaccine
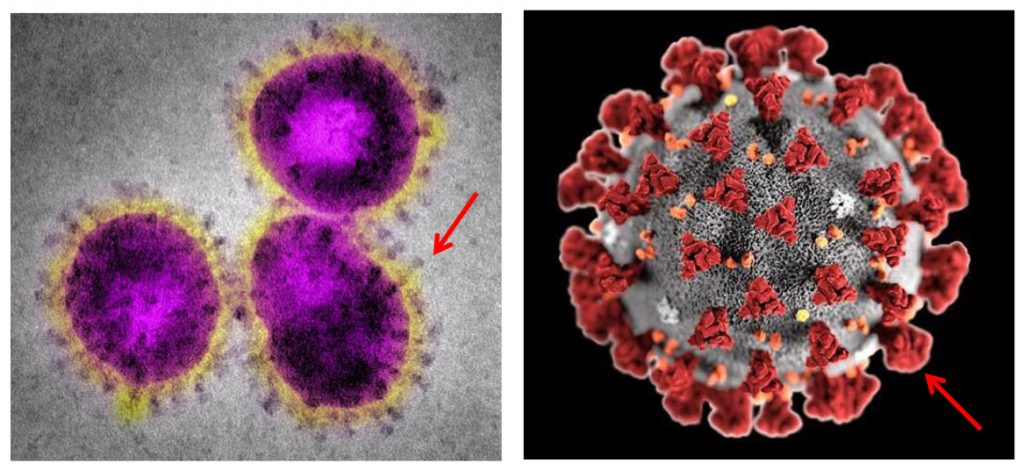
Since the beginning of the COVID-19 pandemic, scientific resources from many countries have devoted themselves to elucidating the molecular mechanisms of infection generated by SARS-CoV-2 and publish their results. After careful review of these studies3,4, our scientific team realized that the virus has glycosyle biomarkers on its surface similar to those on which his scientific team has been focusing its work since 2017.
This discovery prompted our scientific team to get involved in the fight against COVID-19, leveraging its unique expertise in glycan chemistry and the development of fourth-generation synthetic glycan vaccines. A fourth-generation vaccine means that the antigenic component that will stimulate the immune system is first synthesized into a test tube and then attached to a transport protein to form the vaccine to be injected.
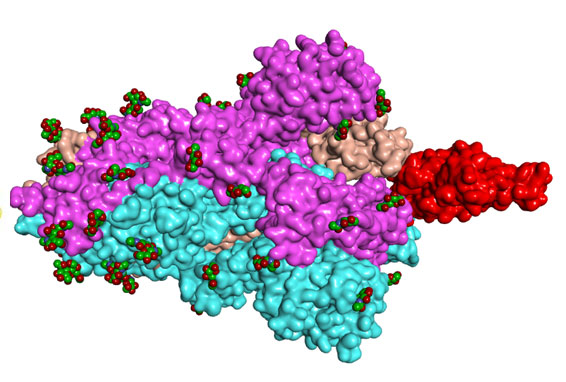
- The preparation of this type of vaccine is safe because staff handle synthetic glycan molecules that are not dangerous, unlike the traditional protocol that uses viruses grown in fermenters.
- Synthetic glycans are chemically pure and their molecular composition is characterized in such a way as to meet GMP (Good Manufacturing Practice) regulatory standards, which serve to ensure greater safety and therapeutic efficiency, while promoting the manufacture of the product in large quantities at low cost.
Next steps
Glycovax Pharma’s goal is to rapidly develop a semi-synthetic vaccine to protect the population from the SARS-C-OoV-2 virus responsible for the COVID-19 pandemic.
Our scientific team has the expertise to create glycoconjugate vaccines. Glycovax co-founder contributed to the development, several years ago, of the first semi-synthetic vaccine against Haemophilus influenzae type B (Hib). This vaccine has been administered to more than 65 million children worldwide and has effectively protected them against the disease.
Glycovax Pharma has the expertise to now produce the immunogenic glycans of SARS-CoV-2 in sufficient quantities for the development of a safe and economical glycoconjugate vaccine.
A first prototype of a semi-synthetic anti-SARS-CoV-2 vaccine is currently in development. With appropriate resources, the Glycovax Pharma team will be able to assess rapidly the effectiveness of this vaccine and determine if it is able to destroy the virus responsible for the COVID-19.
Once the clinical trials will be successful, this vaccine can then be manufactured quickly and on a large scale.
References
Websites
Articles
Visual Capitalists
COVID-19 – Visualizing the History of Pandemics
https://www.visualcapitalist.com/history-of-pandemics-deadliest/
