Vaccines are among the most powerful inventions in history, making once-feared diseases preventable.
WHO Director-General
Dr Tedros Adhanom Ghebreyesus
Source : WHO, 2024
Vaccine design has evolved over the past three centuries, driven by scientific innovation and technological progress, leading to more efficient products and production platforms.
Breakthroughs in glycoscience and immunology have established glycan-based conjugate vaccines as a key technology. These vaccines have a strong safety track record and are highly effective in preventing and protecting against bacterial infections.
Glycovax is redefining capsular polysaccharide conjugate vaccine design to create the next generation of carbohydrate-based vaccines.
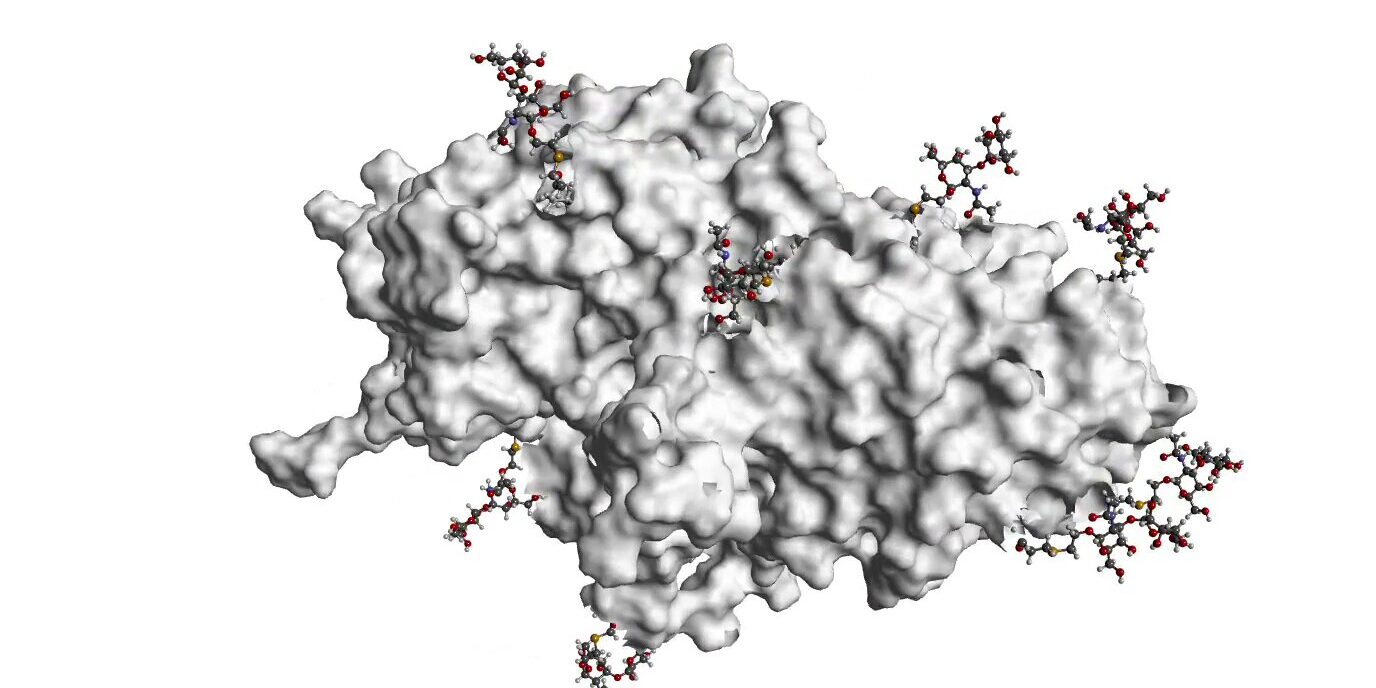
Our technology platform utilizes fully synthetic carbohydrate antigens, providing key advantages over natural antigens, including optimal molecular consistency, precise control over conjugation and presentation, reliable production, the potential for enhanced safety, and broad efficacy.
It combines a customizable linker, the CRM197 carrier protein, and a novel immunostimulating adjuvant to develop cutting-edge vaccines.
With unprecedented flexibility, this platform enables the design of vaccines with optimal reactivity and efficacy, addressing unmet medical needs in infectious diseases and oncology.
Our Technology
GlycoForge
A novel, innovative platform technology designed to accelerate the development of new glycoconjugate vaccines.
Pipeline
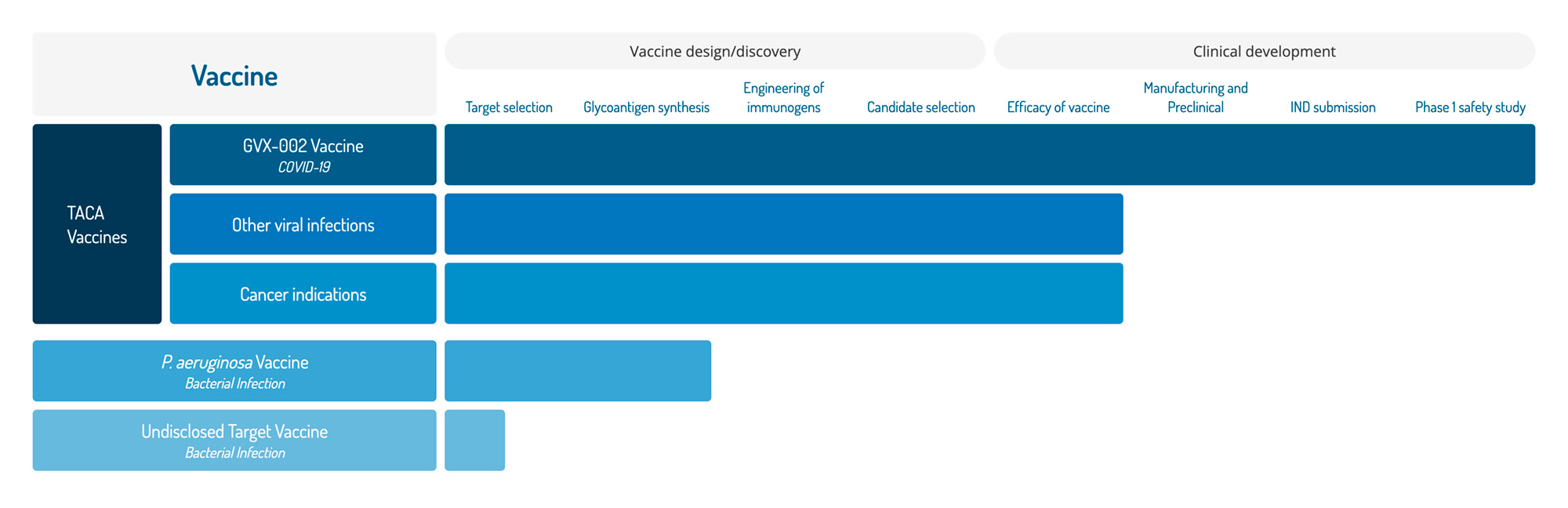
Vaccine
Vaccine design/discovery
TACA
Vaccines
Clinical development
Target selection
Glycoantigen synthesis
Engineering of immunogens
Candidate selection
Efficacy of vaccine
Manufacturing and Preclinical
IND submission
Phase 1 safety study
GVX-002 Vaccine
COVID-19
Other viral infections
Cancer indications
P. aeruginosa Vaccine
Bacterial Infection
Undisclosed Target Vaccine
Bacterial Infection
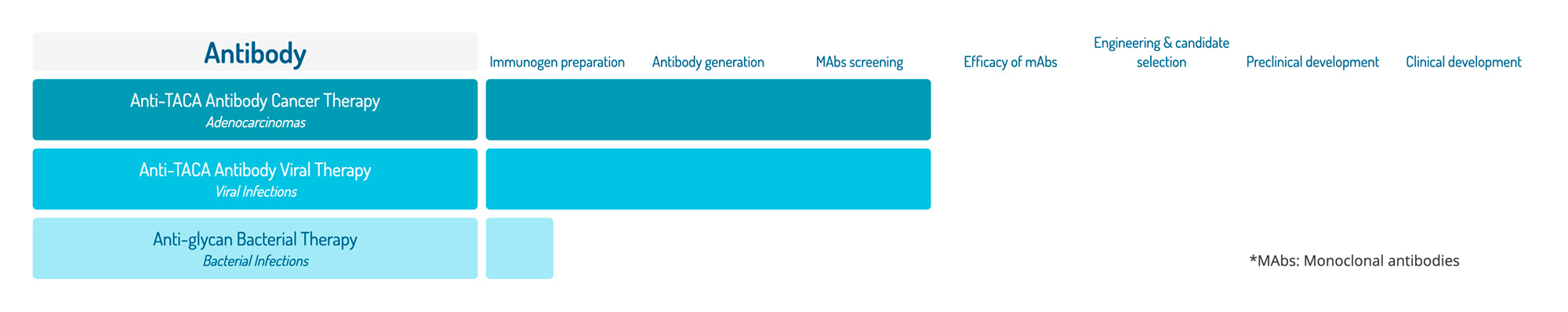
Antibody
Immunogen preparation
Antibody generation
MAbs screening
Efficacy of mAbs
Engineering & candidate selection
Preclinical development
Clinical development
Anti-TACA Antibody Cancer Therapy
Adenocarcinomas
Anti-TACA Antibody Viral Therapy
Viral Infections
Anti-glycan Bacterial Therapy
Bacterial Infections
*MAbs: Monoclonal antibodies
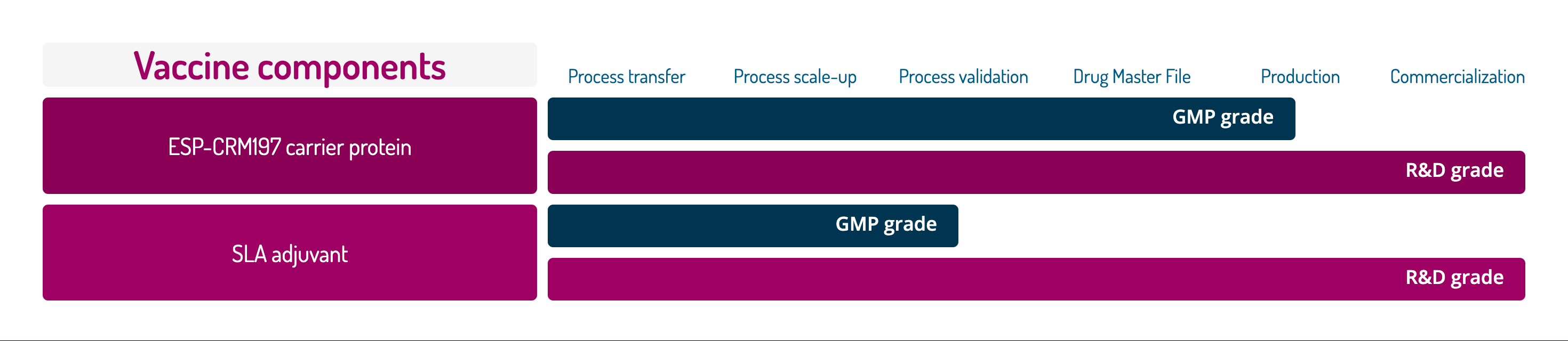
Vaccine components
Process transfer
Process scale-up
Process validation
Drug Master File
Production
Commercialization
ESP-CRM197 carrier protein
GMP grade
R&D grade
SLA adjuvant
GMP grade
R&D grade
Vaccine components
Two vaccine components are produced and commercialized by Glycovax Pharma for vaccine developers.
ESP-CRM197 Carrier Protein
High-Quality, Scalable, Compliant CRM197
ESP-CRM197 is produced to the highest quality standards and offered in multiple grades to meet the needs of vaccine developers at various stages of conjugate development.
It is backed by a Drug Master File (DMF) filed with the US FDA, simplifying the developers’ regulatory submissions.
ESP-CRM197 is produced by Espoir Therapeutics Inc., Glycovax branch dedicated to commercial manufacturing.

SLA Adjuvant
A potent, high-purity adjuvant compatible with various antigens
SLA (sulfated lactosyl archaeol) is a novel adjuvant obtained through a fully synthetic process. Its key advantages include:
- Wide applicability for different vaccine types and routes of administration
- Stimulating a balanced immune response (humoral and cellular)
- Enhancing antibody production
- Reducing the required antigen dose, thereby lowering costs
Soon to be produced under rigorous GMP standards to ensure high purity, SLA is designed for both human and animal immunization, thanks to its potent adjuvant activity.